Monomers
Monomers in Radical Polymerization
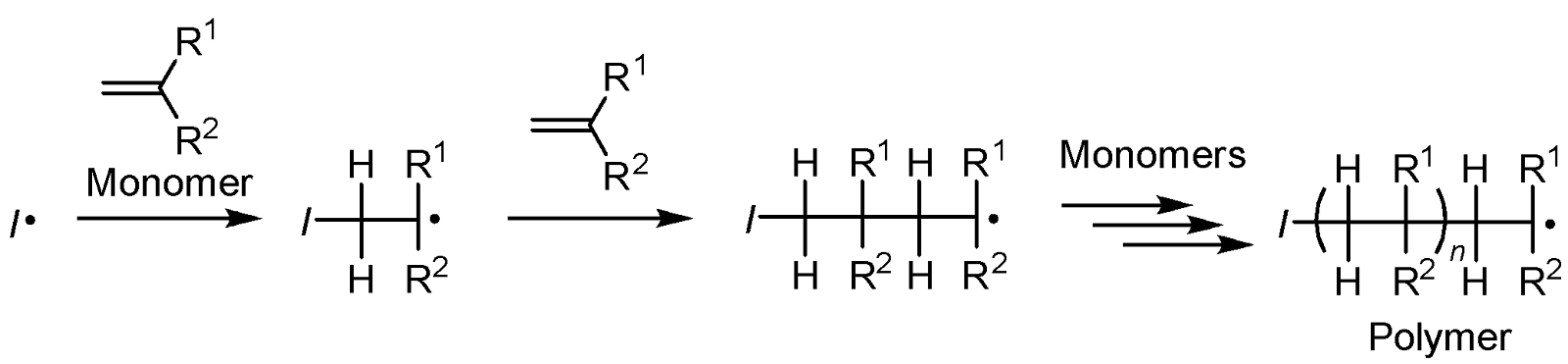
A monomer is the starting material for a polymer and is converted into a polymer by a polymerization reaction. A monomer usually possesses two reaction sites for the bond formations, and the reaction sites and types of functionalities as the reaction sites depend on the polymerization reaction. Mono-substituted (R1 or R2 = H) and 1,1-disubstituted (R1, R2 ≠ H) alkenes are usually used as a monomer in radical polymerization, and an initiating radical (/·) or the polymer-end radical adds to a monomer producing the chain-extended radical species, which further reacts with the next monomer (Figure 1). The repetition of this addition process finally results in polymer formation.
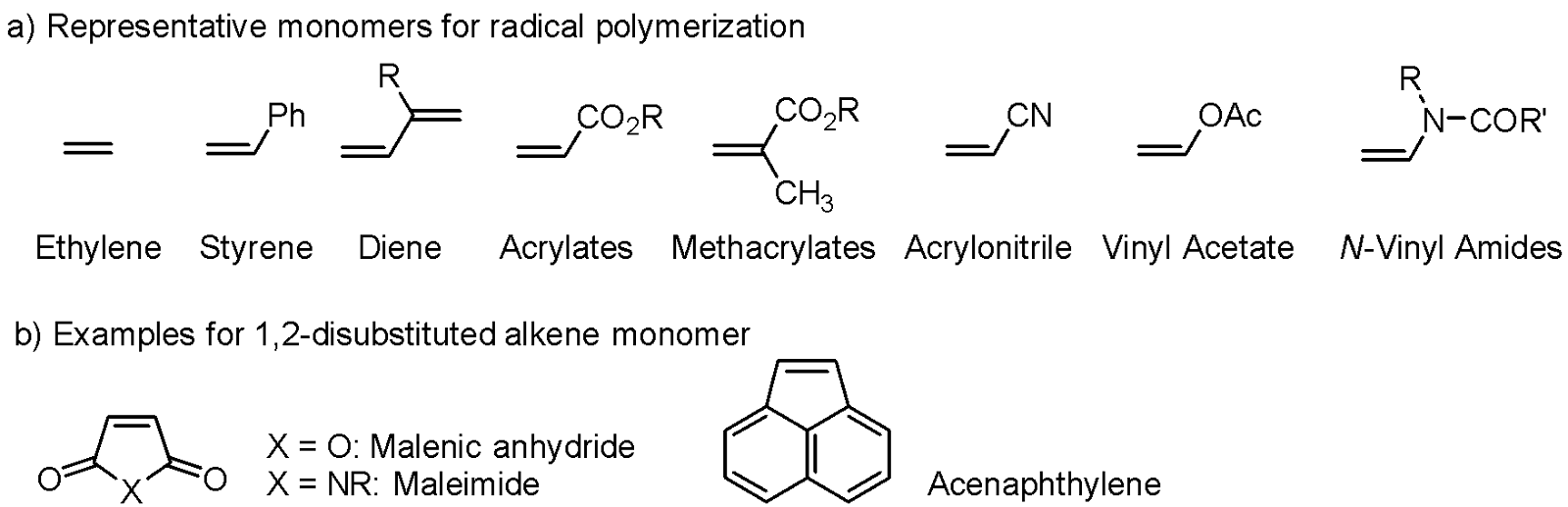
Representative Monomers
Structures of representative monomers used in radical polymerization are shown in Figure 2a. As mentioned above, mono-substituted and 1,1-disubstituted alkenes are typical monomers. Ethylene (R1, R2 = H) can also be used, but polymerization usually requires high-pressure conditions. The reactivity of monomers highly depends on substituents R1 and R2, and monomers are classified as conjugated and non-conjugated monomers depending on the substituents. The monomers bearing conjugating groups with alkene are called conjugated monomers. Styrene, dienes, such as butadiene, acrylates, methacrylates, acrylonitrile, and acrylamides are classified into this class. The other groups are called unconjugated monomers, which do not have conjugating group. Ethylene, vinyl acetate, vinyl chloride, and N-vinyl amides belong to this class.
1,2-Disubstituted alkenes are not usually used as monomers because radical addition is sensitive to steric bulkiness and 1,2-disubstituion significantly retards the addition reaction. However, maleic anhydride, maleimides, and acenaphthylene, which are the 1,2-disubstituted alkenes (Figure 2b), can participate in radical polymerization. Maleimides can be homopolymerized, but maleic anhydrid and acenaphthylene can polymerize only by copolymerization.